
In the laboratory, it is used as a more easily handled replacement for hydrogen peroxide. for germ reduction on contact lens surfaces or as an antiseptic for mouthwashes, ear drops or for superficial wounds and ulcers. Ĭarbamide peroxide is also suitable as a disinfectant, e.g. It is also used to relieve minor inflammation of gums, oral mucosal surfaces and lips including canker sores and dental irritation, and to emulsify and disperse earwax. As a drug, this compound is used in some preparations for the whitening of teeth. Hydrogen peroxide - urea is mainly used as a disinfecting and bleaching agent in cosmetics and pharmaceuticals.

Applications Disinfectant and bleaching agent The solubility of commercial samples varies from 0.05 g/mL to more than 0.6 g/mL. Because of the tendency for thermal decomposition, which accelerates at temperatures above 82 ☌, it should not be heated above 60 ☌, particularly in pure form. Thus the compound is suitable as a safe substitute for the unstable aqueous solution of hydrogen peroxide. So just like hydrogen peroxide, the (erroneously) so-called adduct is an oxidizer but the release at room temperature in the presence of catalysts proceeds in a controlled manner. Upon dissolving in various solvents, the 1:1 complex dissociates back to urea and hydrogen peroxide.

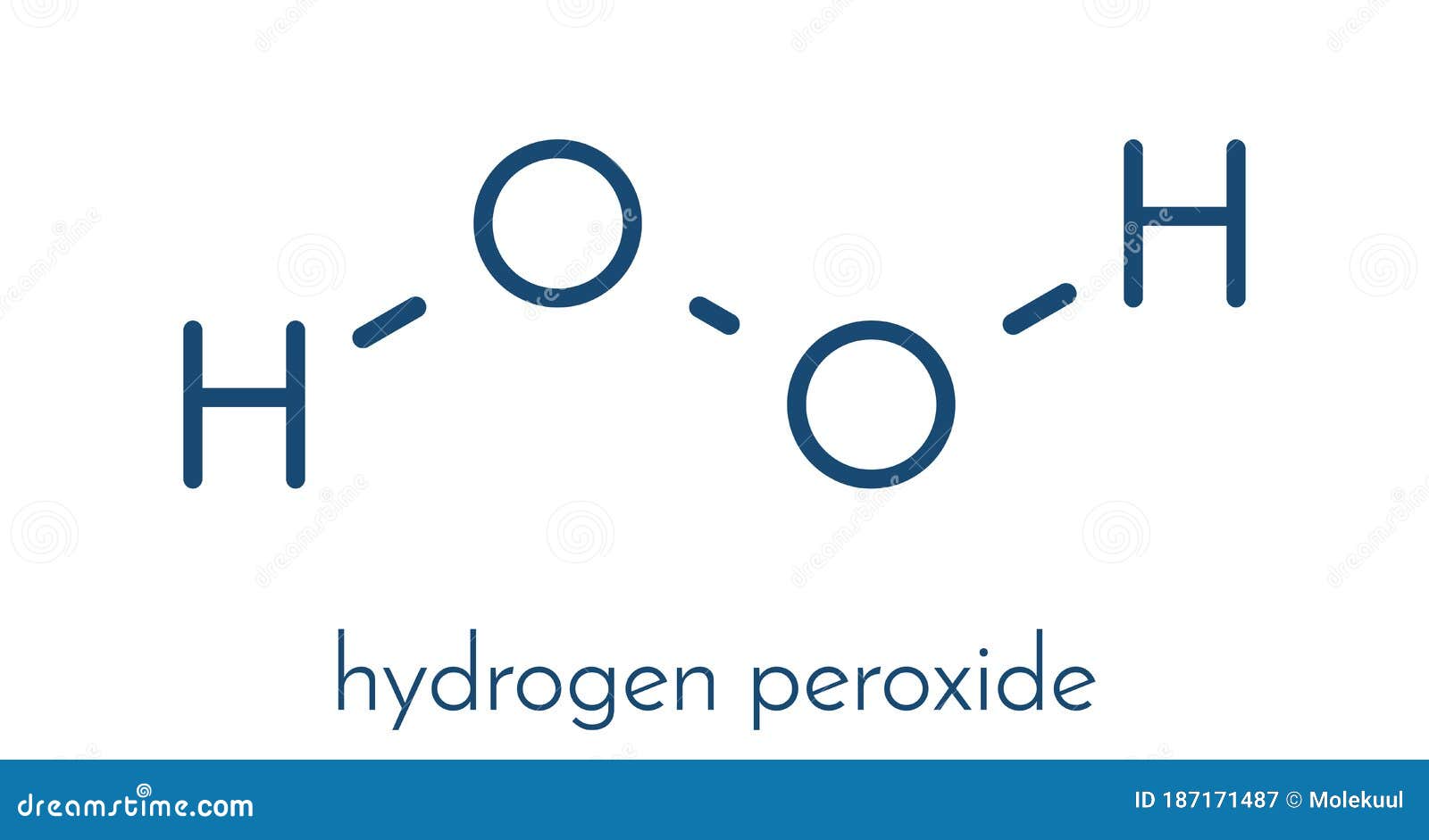
Hydrogen peroxide-urea is a readily water-soluble, odorless, crystalline solid, which is available as white powder or colorless needles or platelets. The solid state structure of this adduct has been determined by neutron diffraction. The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization. The remaining impurity consists of urea.Īkin to water of crystallization, hydrogen peroxide cocrystallizes with urea with the stoichiometry of 1:1. ĭetermination of the hydrogen peroxide content by titration with potassium permanganate solution gives a value of 35.4% which corresponds to 97.8% of the theoretical maximum value. upon cooling this solution, hydrogen peroxide - urea precipitates in the form of small platelets. All rights reserved.For the preparation of the complex, urea is dissolved in 30% hydrogen peroxide (molar ratio 2:3) at temperatures below 60 ☌. On behalf of the United States of America. Shall not be liable for any damage that may result fromįor NIST Standard Reference Data products. However, NIST makes no warranties to that effect, and NIST Uses its best efforts to deliver a high quality copy of theĭatabase and to verify that the data contained therein haveīeen selected on the basis of sound scientific judgment.
The National Institute of Standards and Technology (NIST)
#Chemical formula for hydrogen peroxide professional
NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data).Computational Chemistry Comparison and Benchmark Database.Vibrational and/or electronic energy levels.Use this link for bookmarking this species This structure is also available as a 2d Mol file IUPAC Standard InChIKey: MHAJPDPJQMAIIY-UHFFFAOYSA-N Copy.


 0 kommentar(er)
0 kommentar(er)
